SOP management systems deliver multiple benefits. With an electronic SOP management system you can:
- manage trainings
- use synergies
- use a central web-based system organisation-wide
- have flexible access from inside and outside the organisation
- use a system that requires minimal service
- manage your database efficiently and use non-overlapping data sets
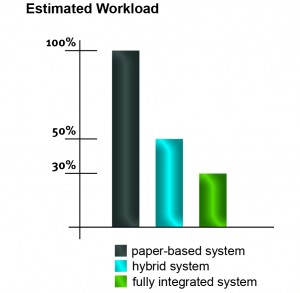
SOP management systems help companies streamline routine tasks such as sending notifications and reminders, versioning of documents and using persisting identifiers. Even hybrid systems provide digital management of documents and processes but a paper-based signature is still required. Please find below a rough estimate of how the amount of workload can be reduced by using an electronic SOP management system.
Workload reduction for the SOP management:
- hybrid system (electronic/paper-based): up to 50%
- fully integrated system (paperless): up to 70%
The use of a SOP management system ensures that high quality audits will be performed as all relevant data is stored centrally. Information can be retrieved at any time, even in the course of an audit. This information can be made available for the management of SOPs, to fill forms and quality documents and for attending trainings.
Quality assurance for GxP audits:
- safe and GxP compliant SOP management
- safe and GxP compliant management of quality forms
- safe and GxP compliant management of trainings
Convince your stakeholders of the quality of your work and enable security for third party. With the SOP management system you can allow carefully selected third parties to access to your data such as customers and investors. KMmaster Life Science Edition can be implemented within available IT environments and allows companies to comply with FDA 21 CFR Part 11 requirements.
Security for third party:
- security towards customers
- security towards investors
- FDA 21 CFR Part 11 compliance
Back to Solution for SOP management.


