 The web-based KMmaster Life Science Edition supports GxP quality processes by providing a fully integrated system for the management of standard operating procedures (SOPs), quality forms and trainings using the qualified electronic signature. KMmaster Life Science Edition was designed to produce and manage standard operating procedures and other types of quality documents including all related information at any time and place.
The web-based KMmaster Life Science Edition supports GxP quality processes by providing a fully integrated system for the management of standard operating procedures (SOPs), quality forms and trainings using the qualified electronic signature. KMmaster Life Science Edition was designed to produce and manage standard operating procedures and other types of quality documents including all related information at any time and place.
With an electronic SOP management system you will:
- have access to valid and reliable documents
- adhere to review cycles (punctuated by reminders and alerts)
- be able to developing quality documents using workflows based on time-related constraints
- use an intelligent search providing fast and accurate search results
- reduce the cost associated with archiving and storage management
- become familiar with new processes and procedures more easily
- be able to provide customers, auditors and inspectors with documents
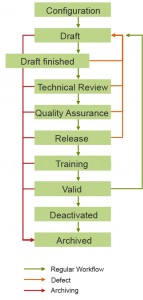
Our software fully complies with GxP standards. KMmaster Life Science Edition provides a workflow for the management of SOPs. Please see the description of the workflow shown in the image above. With a predefined set of roles and permissions for various functions. KMmaster Life Science Edition helps companies to attain and sustain compliance with GxP standards by:
- ensuring adherence to roles and permissions in accordance with GxP regulations
- issuing alerts
- structuring of users into groups and classifying documents into categories
Back to Solution for SOP management


